Terveystalo Biobank and Clinical Research
Terveystalo Biobank and Clinical Research (TBCR) is the largest site management unit in Finland. We have obtained strong knowledge and experience of clinical trials since 1992.
TBCR offers high-quality clinical research services in compliance with ICH-GCP. We currently manage 20 clinical trial sites strategically located across the country, offering the capacity of approximately 360 clinics in about 100 localities with 14,800 healthcare professionals in Terveystalo. There are many national opinion leaders amongst them in several therapeutic areas as well as trained Coordinating Investigators. We have both GCP-compliant investigators and study coordinators.
Apart from clinical drug trials, we can also conduct trials on nutraceuticals and biomaterial studies.
High experience at our client's service
TBCR provides easy access to successful trials:
- free feasibility assessment
- fast site and investigator selection
- fast trial subject prescreening using our huge EMR database
- experienced and GCP-compliant Investigators and Coordinators
- centralised budget and contract negotiations
- opinion leaders in several therapeutic areas
- trained National Coordinating Investigators
- multiple recruitment sources
- Electronic Medical Record (EMR) system in use by all physicians in Terveystalo
- laboratory and imaging services available
- centralised invoicing
| Our values: | Our mission: | Our vision: |
|---|---|---|
| Competence - Caring - Continuous improvement | Clinical trial services in the best interests of human health | TBCR – The leading Site Management Unit in Finland |
Contact information:

Markku Nissilä
Director, Chief Physician, Biobank and Clinical Research
- More than 90 trials ongoing
- Over 1,900 individual trial subjects per year
- Around 10,000 trial related visits per year
- Largest laboratory network in Finland
- The largest site management unit in Finland
- Centralized contract negotiation, budgeting and invoicing
- Search for investigators is free of charge
Terveystalo operates in over 100 localities across Finland
The largest healthcare service company in Finland.
We develop Finnish healthcare by measuring clinical and operational quality and the quality of customer experiences and by introducing new electronic services to the customers.
300 clinics
17 hospitals
40 dental clinics
700,000 people receiving occupational healthcare services
1 200,000 individual customers
over 13,000 healthcare professionals
15% appointments with physicians in Finland
Consenting patients/customers for clinical trials since December 2014
Consent enables Terveystalo to pre-screen and contact potential study subjects and bring them an opportunity to participate in clinical trials.
Consent is a very cost-effective way of recruiting trial subjects.
More than1 400 000 consented customers since 2014
eConsenting customers for biobank since April 2019
- >400 000 biobank consents
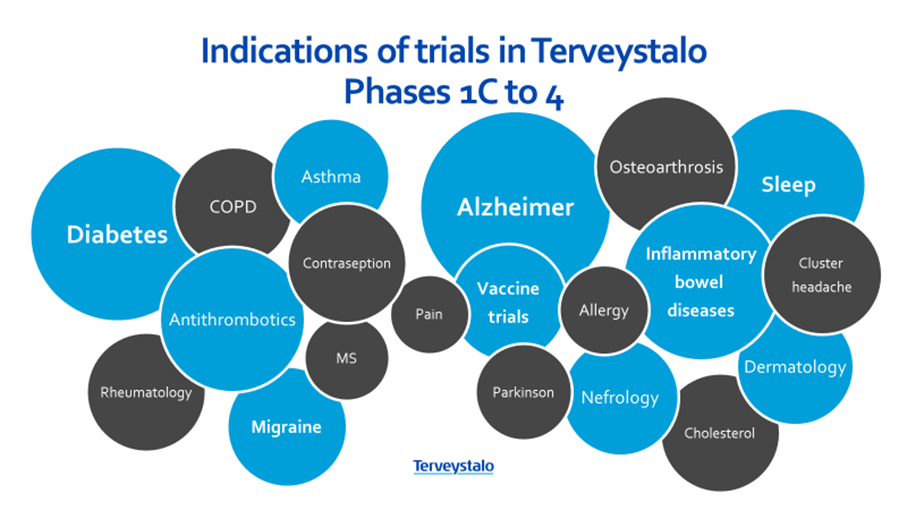
- core interest indications are e.g. asthma, migraine, prediabetes, diabetes, Alzheimer, IBD, MS
Data lake
Pseudonymized data since Jan 2012 Mainly structural data, including:
- ICD-10 diagnoses
- Laboratory analysis parameters
- Demographics
- Medication
- Huge amount of imaging data,MRIs, mammograms etc. in digital format
- 1,2 million individuals per year
- 6,5 million visits per year
- 700 000 occupational healthcarecustomers –of working age